Kotai
Hydrogen Project
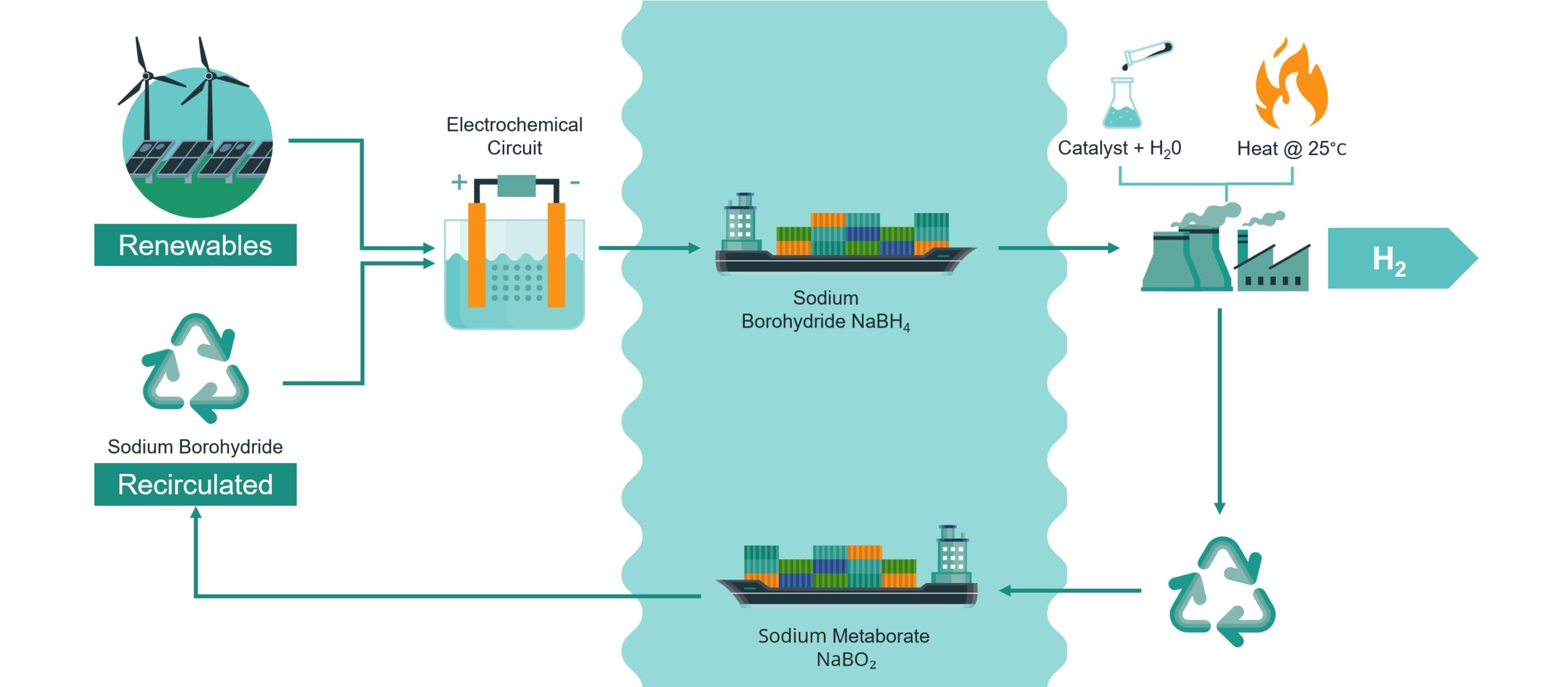
The Kotai Hydrogen Project is a collaboration project with experts from Curtin University in Perth. The research project is investigating the feasibility of using Sodium Borohydride (NaBH4) as a safe ‘carrier’ of hydrogen that can be deployed on demand wherever it is required.
The global hydrogen generation market is expected to grow strongly over the next decade, due to its potential applications:
- Fuel-cell hydrogen for large mobile vehicles
- Shipping and overland transport including trains
- Domestic uses, such as a substitute for natural gas
Whilst hydrogen is viewed as an emissions-free energy source, the cost of generating and transporting hydrogen is limiting its uptake. There are numerous different technologies and plans in the pipeline globally assessing the production of hydrogen. Most of these processes produce hydrogen using low-cost power and then transport the hydrogen to where it is required.
The Kotai Hydrogen Project differs in that the Company is investigating transporting hydrogen in an inert powder form (Sodium Borohydride), which can then be added to water to generate hydrogen (and Sodium Metaborate) wherever hydrogen is required.
The transportation of an inert powder at room temperature and ambient pressures is expected to significantly reduce the transportation costs when compared to transporting hydrogen.
| Transport Temperature | Production Capacity | Transported Cost | |
| Liquid Hydrogen | -253oC | 71kg/m3 H2 | $10/kg H2 |
| Ammonia | -33oC | 106kg/m3 H2 | $8/kg H2 |
| Liquid Organic Hydrogen | Room temperature | 57kg/m3 H2 | $8.50/kg H2 |
| Sodium Borohydride | Room temperature | 137kg/m3 H2 | <$7/kg H2 |
A key part of the research project is investigating whether Sodium Metaborate can be easily and cost-efficiently converted back into Sodium Borohydride, allowing the waste product from the reaction to be renewed.
The Kotai Hydrogen Project is being supported by grants from the ARC Training Centre for the Global Hydrogen Economy, with in kind and cash support from Velox.
High Pressure Release of Hydrogen
With the addition of a catalyst, hydrogen can be chemically compressed during the H2 generation phase high pressure levels up to 1,000 bar possible.
On-site, high pressure hydrogen
Process can generate high pressures on-site, for example, a vehicle fueling station could generate high pressure hydrogen requiring only a closed chamber.
Utilising a recyclable catalyst
Using a catalyst that is readily available and doesn’t deteriorate.
By 2030 Japan is targeting:
- 800,000 fuel cells vehicle
- 1,200 fuel cell buses
- 900 hydrogen fuel stations


